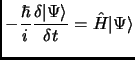 |
(2.1) |
From the fifth postulate in atomic units we have the time dependent Schrödinger equation that describes the evolution of a quantum system.
Under the consideration that
![]() is a stationary state we get the time independent Schrödinger equation
is a stationary state we get the time independent Schrödinger equation
The value ![]() is the energy eigenvalue of the Hamiltonian operator, a scalar that offers the spectrum of the operator.
is the energy eigenvalue of the Hamiltonian operator, a scalar that offers the spectrum of the operator.
The equation 1.4 cannot be solved exactly for a molecular system.
The term
![]() in equation 1.2 does not permit to solve the independent Schrödinger equation
by splitting the problem into a nuclear part separated from an electronic part.
Consequently we will need to solve the equation 1.4
by different stages. This two-stage solution is provided by the Born-Oppenheimer approximation.
in equation 1.2 does not permit to solve the independent Schrödinger equation
by splitting the problem into a nuclear part separated from an electronic part.
Consequently we will need to solve the equation 1.4
by different stages. This two-stage solution is provided by the Born-Oppenheimer approximation.